ICSE Revision Notes for Matter and its Composition Class 9 Chemistry
Chapter Name | Matter and its Composition |
Topics Covered |
|
Related Study |
Matter
- Anything which occupies space or volume, has mass and can be perceived by our senses is called matter.
- All materials are made up of matter. Matter exists in different forms or states.
- Matter in any state is composed of small particles—molecules, atoms or ions.
Properties of Matter
- Matter is made up of tiny particles. These particles have spaces between them, and they attract each other.
- Matter occupies space and has volume and mass.
Characteristics of Particles of Matter
The particles of matter
- Are very small
- Have spaces between them
- Are continuously moving
- Attract each other
Brownian Movement
The random motion of particles suspended in a fluid (liquid or gas) results from their bombardment by the fast-moving atoms or molecules in the fluid (liquid or gas). This haphazard motion of particles is known as Brownian motion.
States of Matter
There are five states of matter
|
Solid State |
Liquid State |
Gaseous State |
|
The space between the particles is very less. |
The space between the particles is slightly more as compared to solids but still very less. The particles of liquid can slip and slide over each other. |
The particles are much farther apart from one another as compared to solids and liquids. Gases have a very disorderly arrangement of particles compared to solids and liquids. |
|
The force of attraction between the particles is strong. Thus, particles in a solid are closely packed. |
The force of attraction between the particles is strong enough to hold the particles together but not strong enough to hold the particles in a fixed position. |
The force of attraction between the particles is negligible; hence, particles of a gas move freely in all directions. Gases can mix or diffuse into other gases. |
|
The kinetic energy of the particles is very less, and thus, solids have an orderly arrangement of particles. Therefore, solids have a fixed shape and a fixed volume. |
The kinetic energy of the particles is more than solids. Thus, liquids have a disorderly arrangement of particles compared to solids. |
The particles have maximum kinetic energy. They move with high speed in all directions and can exert pressure on the walls of its container. |
|
Solids maintain their shape even when they are subjected to external force. i.e. they are rigid. |
Liquids do not have a fixed shape but have a fixed volume. Liquids take up the shape of the container in which they are poured. |
Gases neither have a definite shape nor a definite volume. They fill up the container completely. |
|
Solids cannot be compressed. |
Liquids cannot be compressed much. The compressibility of liquids is almost negligible. |
Gases can be compressed easily. For example, the LPG cylinders used at home and the CNG cylinders used in vehicles. |
Interconversion of States of Matter
The phenomenon of change from one state of matter to another and back to the original state is called interconversion of states of matter.
It is affected by changes in the conditions such as
- Changing the temperature
- Increasing or decreasing the pressure
- Changing both temperature and pressure
- Latent heat: The hidden heat which breaks the forces of attraction between the molecules is known as latent heat. Because the heat energy is hidden in the bulk of the matter, it is called latent heat. The word latent means hidden.
- Latent heat of fusion: The heat energy required to convert 1 kilogram of solid into liquid at its melting point is called latent heat of fusion.
- Latent heat of vaporisation: The heat energy required to convert 1 kilogram of liquid into a vapour or a gas at its boiling point is called latent heat of vaporisation.
Plasma State of Matter
- A highly ionised gas with equal number of positive and negative charges in which the particles exist in super energetic and super excited state is called plasma.
Bose–Einstein Condensate
- In 1920, the Indian physicist Satyendra Nath Bose made a study regarding the fifth state of matter. Based on his study, Albert Einstein predicted a fifth state of matter called the Bose–Einstein condensate.
- The Bose–Einstein condensate (BEC) is formed by cooling a gas of extremely low density, about one hundred thousandth of the density of normal air to super low temperatures.
Change of State of Matter on the basis of Kinetic Theory of Gases
Melting and Freezing
Melting (Solid → Liquid)
- The process of change of state from solid to liquid on absorbing heat at a particular temperature and at one atmospheric pressure is called melting or fusion.
- The constant temperature at which a solid becomes a liquid upon absorbing heat under normal pressure is called the melting point of that solid.
- The melting point increases when the pressure is increased.
Freezing (Liquid → Solid)
- The process of change of matter from the liquid state to the solid state at a particular temperature is called freezing or solidification.
- The constant temperature at which a liquid changes into a solid by giving out heat energy is called the freezing point of that liquid.
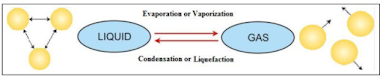
Evaporation and Condensation
Evaporation (Liquid → Gas)- The process of conversion of a substance from the liquid state to the gaseous state at any temperature below its boiling point is called evaporation or vaporisation.
- Evaporation is a surface phenomenon.
Factors affecting evaporation
The rate of evaporation increases with
- Increase in the surface area of the liquid
- Increase in temperature
- Decrease in humidity
- Decrease in pressure
- Increase in wind speed
Boiling
- Boiling is the process of change of a liquid into a vapour at a particular temperature from all parts of the liquid.
- Boiling is a bulk phenomenon.
Condensation (Gas → Liquid)
- The process of change of state of a substance from its gaseous state to its liquid state at a particular temperature is called condensation or liquefaction.
- During condensation, the particles of the gas lose kinetic energy and come closer until they are attracted to each other and form a liquid.
Sublimation (Solid → Gas)
A change of state of a substance directly from solid to gas without changing into a liquid state or vice versa is called sublimation. The common substances which undergo sublimation are camphor, naphthalene, ammonium chloride, solid carbon dioxide and iodine.
Law of Conversation of Mass
In 1774, the law of conservation of mass was proposed by A. Lavoisier. It states that mass can neither be created nor be destroyed in a chemical reaction. The total mass of the reactants is equal to the total mass of the product.
Mass of A + Mass of B = Mass of C + Mass of D
Example:
Thus, the total mass of the reactants is equal to the total mass of the products.