ICSE Revision Notes for Study of Compounds Class 10 Chemistry
Chapter Name | Study of Compounds |
Topics Covered |
|
Related Study |
Hydrogen chloride (HCl)
Hydrogen chloride was first prepared by Glauber in 1648 by heating rock salt with concentrated sulphuric acid.
Occurrence
A small quantity of hydrogen chloride gas is found in free state in gases given out by erupting volcanoes. It is also present in the form of gastric juice in the stomach of mammals.
Preparation
• In laboratory, hydrogen chloride gas is prepared by heating sodium chloride with concentrated sulphuric acid.
This is done by taking sodium chloride (i.e., rock salt) in dry round bottomed flask. The apparatus can be set as shown below. Concentrated H2SO4 is poured from thistle funnel till its lower end is completely immersed in sulphuric acid. Heat the mixture gently.
Do you know ?
Chlorine is a greenish-yellow gas.
Physical Properties of Hydrogen Chloride
- Hydrogen chloride gas is colourless and pungent-smelling with sour taste.
- It has a very irritating odour.
- It is easily liquefied to a colourless liquid. (Boiling point = 189 K)
- It freezes to a white crystalline solid. (Freezing point = 159 K)
- It is extremely soluble in water and ionises as:
HCl(g) + H2O(l) → H2O+(aq) + Cl-(aq) - High value of Ka indicates that it is a strong acid in water.
- Aqueous solution of HCI is called hydrochloric acid.
- Hydrogen chloride gas is extremely soluble in water. It is described using fountain experiment.
Take hydrogen chloride gas in a round bottomed flask and fix a two-holed stopper in its mouth. Through one hole, pass a long jet tube such that the jet is close to the base of flask. Through the other hole, pass a dropper half filled with water. Clamp the flask with iron stand. Place a beaker containing blue litmus solution under the jet tube.
Chemical Properties of Hydrogen Chloride
- Hydrogen chloride is neither combustible nor does it support combustion.
- On heating at above 500°C, it dissociates into hydrogen and chlorine.
- Action with ammonia
On mixing with ammonia gas, it forms dense white fumes due to formation of ammonium chloride.
NH3 + HCl → NH4Cl - Action with indicators
Hydrogen chloride turns
• blue litmus solution red
• orange coloured methyl solution pink
• pink coloured alkaline phenolphthalein solution colourless - Action with active metals
It reacts with metals to form chlorides and hydrogen.
Ca + 2HCl → CaCl2 + H2
It also reacts with metallic oxides, hydroxides, carbonates, sulphides, etc. Hydrochloric acid decomposes salts of weaker acids.
Example:
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
NaHCO3 + HCl → NaCl + H2O + CO2
Na2SO3 + 2HCl → 2NaCl + H2O + SO2
It is extremely soluble in water and ionises as
HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)
Aqua regia is a mixture of 3 parts of concentrated hydrochloric acid and 1 part of concentrated nitric acid. It dissolves noble metals such as Au, Pt, etc.
Au + 4H+ + NO- + 4Cl- → AuCl–4 + NO + 2H2O
3Pt + 16H+ + 4NO-3 + 18Cl- → 3PtCl2-6 + 4NO + 8H2O
Uses of hydrochloric acid
In the manufacture of chlorine, ammonium chloride, and glucose (from corn starch)
- For extracting glue from bones and purifying bone black
- In medicines
- As a laboratory reagent
- For preparing aqua regia
Hydrochloric Acid
The aqueous solution of HCl gas is known as hydrochloric acid.
HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)
Difference between hydrogen chloride and hydrochloric acid:
|
Hydrogen Chloride |
Hydrochloric Acid |
|
Dry and liquid forms do not turn blue litmus paper red, hence it is non-acidic in nature. |
Aqueous solution of HCl gas turns blue litmus paper red, hence it is acidic in nature. |
Laboratory Preparation
Steps:
- Funnel and beaker are arranged as shown in figure:
- HCl gas is passed into the water.
- Since HCl gas is highly soluble in water, the water ascends in the funnel. Back-suction occurs and in turn, the level around the funnel drops making an air gap between the rim of the funnel and the water surface.
- When the pressure inside and outside becomes equal, the water that had risen in the funnel falls down again.
- The process goes on till water is saturated with HCl gas resulting in the formation of hydrochloric acid.
Properties of Hydrochloric Acid
Physical Properties
- Colour: Colourless
- Smell: Pungent choking smell
- Taste: Sour
- Physiological action: Corrosive and cause blister on skin
- Solubility: Soluble in water
- Boiling point: 110° C
Chemical Properties
Nature: It is strongly acidic in nature and that is confirmed by the help of indicators.
|
Indicators |
Original Colour |
Final Colour |
|
Moist litmus |
Blue |
Red |
|
Methyl orange |
Orange |
Pink |
|
Phenolphthalein |
Colourless |
Colourless |
Action on metals: It forms metallic chloride with the release of hydrogen gas.
Mg + 2HCl → MgCl2 + H2
Fe + 2HCl → FeCl2 + H2
Zn + 2HCl → ZnCl2 + H2
Action on oxides and hydroxides: It forms salts and water only.
MgO + 2HCl → MgCl2 + H2O
FeO + 2HCl → FeCl2 + H2O
ZnO + 2HCl → ZnCl2 + H2O
Fe2O3 + 6HCl → 2FeCl3 + 3H2O
CuO + 2HCl → CuCl2 + H2O
NaOH + HCl → NaCl + H2O
Cu(OH)2 + 2HCl → CuCl2 + 2H2O
With salts of weaker acids: it decomposes salts of weaker acids.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
NaHCO3 + 2HCl → NaCl + H2O + CO2
Na2SO3 + 2HCl → 2NaCl + H2O + SO2
NaHSO3 + HCl → NaCl + H2O + SO2
Action on thiosulphates: It produces sulphur dioxide gas, sodium chloride, water and sulphur as yellow ppt. This reaction is used to distinguish thiosulphates and sulphites.
Na2S2O3 + 2HCl → NaCl + H2O + SO2 + S↓
Pb(NO3)2 + 2HCl → PbCl2 + 2HNO3
Hg(NO3)2 + 2HCl → HgCl2 + 2HNO3
Oxidation of hydrochloric acid: It can be easily oxidised by strong oxidising agents like manganese dioxide, lead dioxide and red lead to chlorine.
Formation of aqua regia: With salts of weaker acids: it decomposes salts of weaker acids. When three parts of concentrated HCl and one part of concentrated HNO3 are mixed, aqua regia is formed which is used for dissolving noble metals, e.g., gold, platinum.
Uses of Hydrochloric Acid
- In the laboratory, it acts as a reagent
- In the manufacturing of chlorine, chlorides (NH4Cl), dyes, drugs, glucose from corn starch
- For purifying bone black
- In cleaning metal surface in industries
- In tanning and calico painting industries
- In medicines
Tests for Hydrogen Chloride and Hydrochloric Acid
- When a glass rod dipped in ammonium solution is brought near the vapours of HCl gas, thick white fumes of ammonium chloride are formed.
- Both HCl gas and acid give a white ppt of silver chloride with silver nitrate.
- A greenish-yellow gas, i.e. chlorine, is liberated when conc. HCl is heated with oxidising agent like MnO2. The liberated gas turns moist starch iodine paper blue-black.
Ammonia (NH3)
The composition of ammonia was determined by Claude Berthollet in 1785.
Occurrence
- In the free state, ammonia is found in air and natural water.
- In the combined state, it is obtained by destructive distillation of coal or wood. Also, it is found on the sides of craters and fissures of lava of volcanoes.
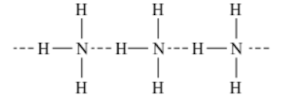
Structure
- It has a trigonal pyramidal structure with nitrogen atom at the apex.
- It has three bond pairs and one lone pair of electrons.
Forms
- Dry ammonia gas (gaseous ammonia)
- Liquid ammonia (liquified ammonia)
- Liquor ammonia fortis (saturated solution of ammonia in water)
- Laboratory bench reagent (dilute solution of liquor ammonia)
Preparation of ammonia
Ammonia can be prepared by the following methods.
Ammonium salts on warming with caustic alkali produce salt, water and ammonia gas as shown in the reactions below:
NH2CONH2 + 2H2O → (NH4)2 CO3 ↔ 2NH3 + H2O + CO2
On a small scale, ammonia is obtained from ammonium salts, which decompose when treated with caustic soda or lime. It forms a metal salt, water, and ammonia gas.
In this method, the mixture of ammonium chloride and dry calcium hydroxide is placed in a round-bottomed flask. It is clamped to an iron stand so that its neck is tilting downwards. This stops water vapours formed by condensation to trickle back into the hot flask.
2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O + CaCl2
(NH4)2SO4 + 2NaOH → 2NH3 + 2H2O + Na2SO4
The ammonia gas contains water vapours as impurities. The water vapour has to be removed as the ammonia gas is extremely soluble in water. Thus, it is passed through drier containing quicklime.
The vapour density of ammonia is 8.5. Therefore, it is lighter than air. Thus, it is collected by downward displacement of the air. Also, since ammonia gas is extremely soluble in water, it cannot be collected over water.
Ammonia can also be prepared by treating metal nitrides like magnesium, sodium and aluminium with warm water.
In this method, magnesium nitride is placed in a conical flask. Warm water is allowed to trickle on it. This evolves moist ammonia gas.
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
The ammonia gas contains water vapours as impurities. The water vapour has to be removed as ammonia gas is extremely soluble in water. Hence, it is passed through drier containing quicklime.
The vapour density of ammonia is 8.5. Therefore, it is lighter than air. Thus, it is collected by downward displacement of air. Also, since ammonia gas is extremely soluble in water, it cannot be collected over water.
The following reactions take place when water reacts with sodium and magnesium nitride.
Na3N + 3H2O → 3NaOH + NH3
AlN + 3H2O → Al(OH)3 + NH3
Preparation of Aqueous ammonia
By dissolving ammonia in water, an aqueous solution of ammonia is obtained. Take water in a container and dip a small portion of the mouth of the funnel in water.
The level of the water decreases when ammonia dissolves in water at a higher rate than that of its production.
The water rushes into the funnel due to the decrease in the pressure above water level and this results in loss of contact between water and the rim of the funnel.
The funnel comes in contact with water again as the water is pushed down by the ammonia produced. This is how ammonia dissolves in water without a back suction.
On large scale, ammonia is obtained by Haber’s process.
A mixture of hydrogen and nitrogen gases in the ratio 3:1 is taken in the compressor. It is then compressed from 200 atm to 900 atm pressure and passed over the heated catalyst in catalyst chamber.
The mixture is maintained at a temperature between 450 − 500°C. In the condenser, the hot mixture of ammonia gas and unreacted hydrogen and nitrogen gases coming out of catalyst chamber are led to cooling pipes.
N2(g) + 3H2(g) ↔ 2NH3(g) ; ∆f Hϴ = - 46 .1 kJ mol-1
The reaction is reversible and exothermic in nature.
Catalysts such as iron oxide with small amounts of molybdenum are used to increase the rate of attainment of equilibrium.
High pressure favours the formation of NH3.Optimum condition
Pressure = 200 × 105 Pa(about 200 atm)
Temperature ∼ 700 K
Finely divided iron as catalyst and molybdenum or Al2O3 as promoter increase the rate of reaction
The produced ammonia gas is collected by either liquefaction as ammonia has higher boiling point than nitrogen and hydrogen hence, it condenses easily, or absorption in water as ammonia is highly soluble in water. The unused mixture of hydrogen and nitrogen gases are recompressed and then recycled into catalyst chamber.
Properties of ammonia
- It is a colourless non-poisonous gas with a characteristic pungent odour. It is lighter than air and extremely soluble in water because of hydrogen bonding.
- It can be liquefied when cooled to 10° C under pressure of 6 atm. It forms white crystals on cooling.
- 3Ammonia has higher melting point and boiling point, latent heat of vaporisation or fusion because of its ability to form hydrogen bonding with itself. Since it has third highest electronegativity so it can form hydrogen bonds with itself and also with water.
- It has basic nature because of the presence of a lone pair of electrons.
- It acts as a reducing agent
3CuO + 2NH3 → 3 Cu + N2 + 3H2O - It is lighter than air with the vapour density of 8.5.
- Inhaling this gas causes irritation to the eyes and respiratory system.
- It is highly soluble in water.
Uses
- Due to high dielectric constant, ammonia is a good solvent for ionic compounds.
- It is used as a cleaning agent for removing grease in dry cleaning.
- It is used in the manufacturing of artificial silk.
- It is used as a laboratory reagent.
Properties of Ammonia
Physical properties of ammonia are given below:
- Ammonia is a colourless gas with a pungent odour.
- Freezing point of ammonia = 198.4 K
- Boiling point of ammonia = 239.7 K
- Its vapour density is 8.5 and thus, it is lighter than air.
- Ammonia is extremely soluble in water. It is described using fountain experiment.
- Take ammonia gas in a round-bottomed flask and fix a two-holed stopper in its mouth. Through one hole, pass a long jet tube such that the jet is close to the base of the flask.
- Through the other hole, pass a dropper half filled with water. Clamp the flask with iron stand. Place a beaker containing red litmus solution under the jet tube.
- Press the bulb of the dropper. You will notice that red litmus solution rises up in the jet tube and comes out of the nozzle with a great force and forms a blue coloured fountain.
- The fountain is formed because when the water is introduced from the dropper, it completely absorbs ammonia gas, thus creating a pressure within the flask. To make up for this, red litmus solution rises up. The colour change is due to its basic character.
- In solids and liquids, ammonia is associated with hydrogen bonds and hence, it has higher melting and boiling points.
Chemical properties
Ammonia decomposes at high temperatures or by electric sparks into hydrogen and nitrogen gas.
2 NH3 ⇌ N2 + 3 H2
Ammonia forms ammonium salts with acids. (Example: NH4Cl, (NH4)2 SO4, NH4NO3 etc.)
An aqueous solution of ammonia reacts with hydrochloric acid, sulphuric acid and nitric acid to form ammonium chloride, ammonium sulphate and ammonium nitrate respectively.
NH4OH + HCl → NH4Cl + H2O
2NH4OH + H2SO4 → (NH4)2SO4 + 2H2O
NH4OH + HNO3 → NH4NO3 + H2O
Oxidation of ammonia in excess of oxygen without catalyst
Set up the apparatus as shown. Pass ammonia gas and try to light it. You will observe that the gas does not burn.
Stop passing ammonia and pour oxygen from tube B. Pass ammonia gas and try to light it. You will observe that the gas burns with a yellow flame.
Catalysed oxidation
Pass the electric current through the platinum filament and pass the mixture of ammonia and oxygen gas.
4NH3 + 5O2 → 4NO + 6H2O
2NO + O2 → 2NO2
Stop passing current. You will observe that the platinum filament keeps glowing and red-brown fumes keep forming.
Here, platinum acts as a catalyst.
Reducing property of ammonia gas
Reduction of chlorine
When chlorine is in excess as compared to ammonia, the ammonia reduces chlorine to hydrochloric acid. However, if ammonia is in excess as compared to chlorine, then ammonium chloride is formed.
NH3 + 3Cl2 → 3HCl + NCl3
8NH3 + 3Cl2 → 6NH4Cl + N2
Reduction of metal oxides
Ammonia reduces oxides of less active metal to metal, water, and nitrogen.
3CuO + 2NH3 → 3Cu + 3H2O + N2
Aqueous ammonia
All soluble salts of metals react with aqueous ammonia to form their respective insoluble hydroxide and ammonium salts.
Zn(NO3)2 + 2NH4OH → 2NH4NO3 + Zn(OH)2
Pb(NO3)2 + 2NH4NO3 + Pb(OH)2
FeSO4 + 2NH4OH → (NH4)2SO4 + Fe(OH)2
Being a weak base, it precipitates the hydroxides of many metals to form their salt solutions.
Examples:
ZnSO4(aq) + 2NH4OH(aq) → Zn(OH)2(s) + (NH2)SO4(aq)
(white ppt)
FeCl3(aq) + 3NH4OH(aq) → Fe2O3.xH2O(s) + 3NH4Cl(aq)
(brown ppt)
The Lone pair of electrons on the N-atom makes the NH3 molecule a Lewis base.
It donates the electron pair and forms linkage with metal ions.
Reaction with carbon dioxide: Ammonia forms urea on reaction with carbon dioxide at 150oC and 150 atm.
Test for Ammonia Gas
Odour: Sharp pungent odour
Action on indicators:
- Red litmus turns blue
- Moist turmeric paper turns brown
- Phenolphthalein solution turns pink
Reaction with HCl: Dense white fumes of ammonium chloride are formed
- Affect on copper sulphate solution: Blue precipitate is formed which dissolves and forms a deep blue solution on passing excess of the gas
- Reaction with Nessler's reagent: Ammonium salts turns Nessler's reagent brown
Uses
- Ammonia is used in the production of various nitrogen fertilizers (ammonium nitrate, urea, ammonium phosphate, and ammonium sulphate).
- It is used in the manufacture of some inorganic nitrogen compounds (for example − HNO3) and synthetic products.
- Liquid ammonia is also used as a refrigerant due to the rapid cooling effect produced by quick evaporation of liquid ammonia.
- Environment compatibility
- Efficient use of electricity
- Ease of identification in case of any leak
- Cannot be used in systems with copper pipes as ammonia reacts with copper
- High concentrations of the gas can cause health problems
- It is used in qualitative analysis as a laboratory reagent as it forms coloured metallic hydroxide precipitates.
- It is used to remove grease and perspiration stains from clothes.
- It is used in cleaning tiles, windows etc.
Nitric Acid
- It was called aqua fortis meaning strong water by alchemists in the 8th century. It was first prepared by Glauber in 1650.
- Traces of nitric acid in the free state occur in the air. In the combined state, it occurs as mineral nitrates. Examples include sodium nitrate or Chile saltpetre and potassium nitrate or Bengal saltpetre.
Structure
Nitric Acid in AtmosphereThe following methods are some ways by which nitric acid is formed in the atmosphere:
By lightning discharge: It combines the nitrogen and oxygen present in air to form nitric oxide. This oxide is further oxidised to nitrogen dioxide. The nitrogen dioxide then combines with water vapour and oxygen present in the atmosphere to form nitric acid. This gets washed down to Earth during rains in the form of acid rain.
N2 + O2 → 2NO
2NO + O2 → 2NO2
4NO2 + 2H2O + O2 → 4HNO3
The atmospheric nitrogen is converted to nitrogenous compounds in soil by bacteria, this process is called nitrogen fixation.
Preparation
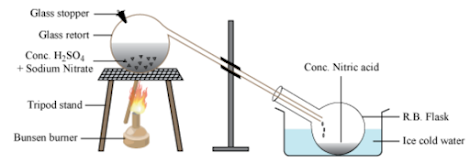
Nitric acid is prepared in the laboratory by distilling equal parts of the mixture of sodium nitrate or potassium nitrate with concentrated sulphuric acid.
Equal parts of sodium nitrate or potassium nitrate mixed with concentrated sulphuric acid are placed in glass retort. It is kept in a round-bottomed flask, which is kept in ice water. The reaction mixture is heated.
NaNO3 + H2SO4 → NaHSO4 + HNO3
The nitric acid formed vaporises immediately and distils into the round-bottomed flask. The nitric acid collected is yellow-brown in colour. It can be removed by passing dry air through nitric acid.
On a large scale, nitric acid is prepared by Ostwald’s process.
The steps involved are:
- Catalytic oxidation of ammonia
- NO formed is recycled and aqueous HNO3 can be concentrated up to ∼ 68% (by mass) by distillation.
- 98% concentration of HNO3 is attained by dehydration with concentrated H2SO4.
Physical Properties of nitric acid
- Nitric acid is a colourless, sour tasting liquid with suffocating smell. (Freezing point = 231.4 K and boiling point = 355.6 K)
- Laboratory grade nitric acid is 68% of the HNO3 by mass and has a specific gravity of 1.504.
- It is highly soluble in water.
- It is non-poisonous but highly corrosive in nature.
Chemical Properties of nitric acid
- Nitric acid behaves as a strong acid in aqueous solution.
- Pure nitric acid is unstable towards heat and decomposes to form nitrogen dioxide.
- Metal oxides, hydroxides, carbonates, and hydrogen carbonates react with dilute nitric acid to form their respective soluble metallic nitrates.
K2O + 2HNO3 → 2KNO3 + H2O
KOH + 2HNO3 → KNO3 + H2O
K2CO3 + 2HNO3 → 2KNO3 + H2O + CO2
KHCO3 + HNO3 → KNO3 + H2O + CO2
Oxidising property of nitric acid
Nitric acid decomposes to liberate nascent oxygen. The nascent oxygen acts as an acceptor of electrons and makes nitric acid a strong oxidising agent.
2HNO3 → H2O + 2NO2 + [O]
Oxidation of metals:
Nitric acid can oxidise most metals (except noble metals such as Au and Pt) to form respective salts and hydrogen gases. The nature of products formed depends upon concentration and temperature of acid.
Action of nitric acid with copper:
3Cu + 8HNO3 (dilute) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3 (conc.) → Cu(NO3)2 + 2NO2 + 2H2O
Action of nitric acid with zinc:
4Zn + 10HNO3 (dilute) → 4Zn(NO3)2 + 5H2O + N2O
Zn + 4HNO3 (conc) → Zn(NO3)2 + 2H2O + 2NO2
Chromium and aluminium do not dissolve in concentrated HNO3 because of the formation of a passive film of oxide on the surface.
Magnesium and manganese react with very dilute nitric acid at room temperature to give respective nitrates and evolve hydrogen gas.
Mg + 2HNO3 → Mg(NO3)2 + H2
Mn + 2HNO3 → Mn(NO3)2 + H2
Oxidation of non-metals:
Non-metals such as carbon and sulphur, on boiling with conc. nitric acid, form their oxides or oxy-acids.
I2 + 10HNO3 → 2HIO3 + 10NO2 + 4H2O
C + 4HNO3 → CO2 + 2H2O + 4NO2
S8 + 48HNO3 (conc.) → 8H2SO4 + 48NO2 + 16H2O
P4 + 20HNO3 (conc.) → 4H3PO4 + 20NO2 + 4H2O
|
Brown Ring Test It is a test for nitrates. It depends upon the ability of Fe2+ to reduce nitrate to nitric oxide (NO). NO then reacts with Fe2+ to form a brown-coloured complex. Procedure Dilute FeSO4 solution is added to an aqueous solution of nitrate ion. After that, concentrated H2SO4 is added along the sides of the test tube. Result A brown ring is observed at the interface between the solution and H2SO4 layers → Indicates the presence of nitrate ion in the solution. |
Tests for nitric acid and nitrates
Aqua regia is capable of reacting with noble metals to form their chlorides.
Au + 3[Cl] → AuCl3
Action of nitric acid on metallic sulphites and bisulphites:
K2SO3 + 2HNO3 → 2KNO3 + H2O + SO2
Ca(HSO3)2 + 2HNO3 → Ca(NO3)2 + 2H2O + 2SO2
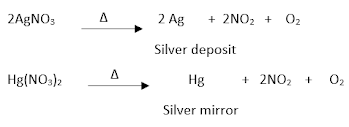
Action of heat on nitrates:
Effect on alkali metal nitrates:
Effect on all other nitrates:
Effect on silver and mercury nitrates:
Effect on ammonium nitrate:
Uses of nitric acid
- It is used industrially in the manufacturing of explosives such as trinitrotoluene, etc.
- It is used in the manufacturing of fertilizers, dyes, drugs, synthetic fibres, etc.
- It is also used for purification of gold, in rocket fuel, and as a laboratory agent.
Oxo Acids of Sulphur
- Concentrated sulphuric acid is known as oil of vitriol. It is also known as the King of Chemicals due to its vast used in industries.
- Structures of some important oxoacids of sulphur:
- Found in hot water sulphur springs
- In combined state:
- Barytes, BasO4
- Gypsum, CaSO4.2H2O
- Kieserite, MgSO4.H2O
Preparation of Sulphuric Acid
2SO2 + 2H2O + O2 → 2H2SO4
SO2 + 2H2O + Cl2 → H2SO4 + 2HCl
SO2 + 2H2O + Br2 → H2SO4 + 2HBr
Reaction between S and concentrated HNO3
S + 6 HNO3 → H2SO4 + 6O2 + 2H2O
Dissolution of sulphuryl chloride in water
SO2 Cl2 + 2H2O → H2SO4 + 2HCl
Manufacture of Sulphuric acid by Contact Process
In contact process, various steps are involved:
Burning of a pure and dry mixture of two parts of sulphur or sulphide ores and one part of air to produce sulphur dioxide
Conversion of sulphur dioxide to sulphur trioxide by the reaction with O2 in presence of vanadium pentoxide or platinised asbestos as catalyst
|
Favourable Condition for Conversion of SO2 to SO3 |
|
It is an exothermic reaction; so maintaining a temperature of 410 - 450° provides good yield. A pressure of 1-2 atm is used. An excess of oxygen increases the yield of SO3. V2O5 is used as a suitable catalyst to speed up the conversion. |
Absorption of sulphur trioxide in H2SO4 to produce pyrosulphuric acid or H2S2O7 (oleum)
Dilution of oleum (H2S2O7) with water gives sulphuric acid (H2SO4) of the desired concentration.
Flow diagram for the manufacture of H2SO4 is given in the figure below.
The plant is operated at a pressure of 2 bar and a temperature of 720 K.
H2SO4 obtained by this process is 96 − 98% pure.
Physical properties of sulphuric acid
- It is a colourless, odourless, and dense liquid.
- It is an oily liquid with specific gravity of 1.84 at 298 K.
- It freezes at 283 K and boils at 611 K.
- It dissolves in water with the evolution of large quantity of heat.
Hence, the concentrated acid must be added slowly in water with constant stirring.
Chemical properties of sulphuric acid
Chemical reactions of H2SO4 are because of its
- low volatility
- strong acidic character
- strong affinity for water
- ability to act as an oxidising agent
In aqueous solution, H2SO4 ionises as:
H2SO4(aq) + H2O(l) → H3O+(aq) + HSO-4(aq) ; Ka1 = Very large (> 10)
HSO-4 + H2O (l) → H3O (aq) + SO2-4(aq) ; Ka2 = 1.2 × 10−2
Since Ka1 > 10, H2SO4 is largely dissociated into H+ and HSO- 4
Greater the value of dissociation constant, stronger is the acid. Therefore, it is a strong acid.
It forms two series of salts:
Normal sulphates (Example: Na2SO4, CuSO4)
Acid sulphate (Example: NaHSO4)
Dilute sulphuric acid reacts with active metals, metal oxides, metal hydroxides, metal carbonates, metal bicarbonates, metal sulphites, metal bisulphites and metal sulphides to form their respective metal sulphates and acid sulphates.
Mg + HgSO4 (dil.) → MgSO4 + H2
K2O + H2SO4 (dil.) → K2SO4 + H2O
NaOH + H2SO4 (dil.) → NaHSO4 + H2O
Na2CO3 + H2SO4 (dil.) → Na2SO4 + H2O + CO2
Na2SO3 + H2SO4 (dil.) → Na2SO4 + H2O + SO2
2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2
2NaHSO3 + H2SO4 → Na2SO4 + 2H2O + 2SO2
Na2S + H2SO4 → Na2SO4 + H2S
Because of low volatility, it can be used for the manufacture of more volatile acids from their corresponding salts.
2MX + H2SO4 → 2HX + M2SO4 (X = F, Cl, NO3) (M = metal)
Concentrated sulphuric acid is a non-volatile acid. It reacts with salts of volatile acids (HCl, HNO3, CH3COOH) to form volatile acids.
NaCl + H2SO4 → NaHSO4 + HCl
NaNO3 + H2SO4 → NaHSO4 + HNO3
CH3COONa + H2SO4 → NaHSO4 + CH3COOH
Concentrated sulphuric acid is a strong oxidising agent as it produces nascent oxygen on thermal decomposition. This nascent oxygen is capable of oxidising metals, non-metals and inorganic compounds to evolve sulphur dioxide gas.
Cu + 2H2SO4 → CuSO4 + 2H2O + SO2
Zn + 2H2SO4 → ZnSO4 + 2H2O + SO2
C + 2H2SO4 → CO2 + 2H2O + 2SO2
S + 2H2SO4 → 3SO2 + 2H2O
2P + 5H2SO4 → 2H3PO4 + 2H2O + 5SO2
2HBr + H2SO4 → Br2 + 2H2O + SO2
H2S + H2SO4 → S + 2H2O + SO2
It is a strong dehydrating agent because of its great affinity towards water. It extracts water from organic acids, carbohydrates and crystallised salts.
Do you know what a dehydrating agent is?
- A substance which removes atoms of hydrogen and oxygen in the form of water from the chemical composition of a substance is called dehydrating agent.
- Because of its strong affinity for water, sulphuric acid removes water from hydrated salts and organic compounds (it is evident by charring action on carbohydrates).
- Dehydration of glucose and sugar occurs with formation of a black porous mass of carbon.
- Concentrated sulphuric acid is a moderately strong oxidising agent and can oxidise both metals and non-metals.
- You know that oxidation takes place either on the addition of oxygen or removal of hydrogen. Sulphuric acid undergoes thermal decomposition and liberates nascent oxygen, which oxidises metals and non-metals.
Cu + 2H2SO4 (conc.) → CuSO4 + SO2 + 2H2O
3S + 2H2SO4 (conc.) → SO2 + 2H2O
C + 2H2SO4 (conc.) → CO2 + 2SO2 + 2H2O - Concentrated sulphuric acid has the ability to precipitate out the siluble sulphates of lead, barium, calcium from the aqueous solution of their salts.
Pb(NO3)2 + H2SO4 → PbSO4 + 2HNO3
BaCl2 + H2SO4 → BaSO4 + 2HCl
Difference Between Dilute and Concentrated Sulphuric Acid
|
Dilute H2SO4 |
Concentrated H2SO4 |
|
Ionises almost completely hence behaves as a strong acid |
Does not ionise completely hence behaves as a weak acid |
|
Strong electrolyte |
Weak electrolyte |
|
Not an oxidising agent |
Good oxidising agent |
|
Not a dehydrating agent |
Good dehydrating agent |
Uses of sulphuric acid
- As an important industrial chemical
- In the manufacture of fertilisers (e.g., ammonium sulphate, superphosphate)
- In petroleum refining
- In detergent industry
- In the manufacture of pigments, paints, and dyestuff intermediates
- In metallurgical applications (such as in cleansing, electroplating, galvanising)
- In the manufacture of nitrocellulose products
- As a laboratory reagent
- In storage batteries