ICSE Revision Notes for Modern Physics Class 10 Physics
Chapter Name | Modern Physics |
Topics Covered |
|
Related Study |
Introduction
- An atom consists of a positively charged nucleus surrounded by electrons revolving around the nucleus in different orbits of some definite radii.
- The neutrons are uncharged particles.
- The atom is electrically neutral.
Structure of Atom
- An atom consists of a nucleus at its centre, surrounded by electrons which are revolving in some specific stationary shells (or orbits).
- Electrons in different shells have different energy values.
- These shells can accommodate at the most 2, 8, 18, 32, 50, 72, 98... electrons.
- The maximum number of electrons in a shell of number n is given as 2n2.
- The size of an atom is determined by the radius of the shell of its outermost electron, and it is of the order of 10−10 m.
- The electron has a negative charge equal to −1·6 × 10−19 C, and its mass is estimated to be 9·1 × 10−31 kg which is approximately 1/1840 times the mass of a proton.
Structure of Nucleus
- The nucleus at the centre of an atom, whose size is of the order of 10−15 m to 10−14 m, consists of protons and neutrons.
- The number of protons in the nucleus is called the atomic number of the element, and it is denoted by the symbol Z.
- The protons and neutrons which are the main constituents of the nucleus are called nucleons. The total number of nucleons in the nucleus is called the mass number of the element, and it is denoted by the symbol A.
Atomic Model
An atom is electrically neutral, and therefore, the number of protons in the nucleus of an atom is equal to the number of electrons revolving around the nucleus of the atom.
Atomic Number
It is the number of protons or electrons in the nucleus of an atom.
Mass Number
It is the total number of nucleons, i.e. number of protons and neutrons, in the nucleus of an atom. The atom is specified by the symbol AZX, where X is the chemical symbol for the element.
Isotopes
- The atoms belonging to the same element with the same atomic number Z but differing in their mass number A are called isotopes.
- The atoms of isotopes have the same number of protons (Z) but different number of neutrons (A − Z) in their nuclei. Because they have the same number of electrons outside the nucleus, their chemical properties are also the same.
- Two kinds of isotopes of some elements:
i. Stable isotopes which have the number of neutrons nearly equal to the number of protons in their nuclei.
ii. Unstable or radioactive isotopes which undergo radioactive decay and are of great medical and industrial use. They have more neutrons than protons in their nuclei.
Example:
- The atoms of different elements which have the same mass number A but differ in their atomic number Z are called isobars.
- The atoms of isobars have the same number of nucleons (A) in their nuclei, but different number of protons (Z) and different number of neutrons (A − Z). The number of electrons outside the nucleus is always equal to the number of protons, so isobars have different number of electrons.
Example:
- The atoms with different number of protons but the same number of neutrons, i.e. different Z and A, but same A − Z, are called isotones. They have different number of electrons.
Example:
Radioactivity
- The substances which disintegrate (or decay) by the spontaneous emission of radiations are called radioactive substances.
Examples: Uranium, radium, polonium, thorium, actinium etc. - The isotopes of nearly all the elements of atomic number higher than 82 (i.e. lead) are radioactive. These are called natural radioactive substances.
- The phenomenon of radioactivity cannot be due to the orbital electrons which could easily be affected by such changes. It should therefore be the property of the nucleus.
- Thus, radioactivity is a nuclear phenomenon. It is the process of spontaneous emission of α, β and γ radiations from the nuclei of atoms during their decay.
Radioactivity as Emission of Alpha, Beta and Gamma Radiations
- Rutherford experimentally found that on subjecting the radiations given out by a radioactive substance to a magnetic field in a direction perpendicular to their path, they separate out into three distinct constituents.
- Those which turn to the left (as given by Fleming’s left-hand rule) must be positively charged and are called alpha (α) particles.
- Those which turn to the right must be negatively charged and are called beta (β) particles. The β particles are deviated more than the α particles.
- Those which pass undeviated must be uncharged (or neutral) and are called gamma (γ) radiations. γ-radiations are electromagnetic waves similar to light and are therefore not affected by the magnetic field.
- Similarly, if the radiations given out by a radioactive substance are subjected to an electric field in a direction perpendicular to their path, they again separate out into three constituents.
Properties of Alpha Particles
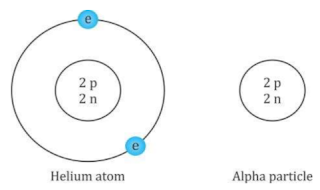
- An alpha particle consists of two protons and two neutrons. It is the same as a doubly ionised helium atom. It is represented as
- The mass of an alpha particle is roughly four times the mass of a proton, and its charge is twice the charge of a proton.
- Their speed is of the order of 107 m s−1.
- An alpha particle strongly ionises the gas through which it passes. The ionising power of α-particles is roughly 100 times that of β-particles and roughly 104times that of γ-radiation.
- An α-particle rapidly loses its energy as it moves through a medium and therefore its penetrating power is quite small. Its penetrating power is roughly 1/100 times that of a β-particle and 10−4times that of γ-radiation.
- They are positively charged, so they are deflected by electric and magnetic fields.
- They affect a photographic plate.
- They cause fluorescence on striking a fluorescent material.
- They destroy the living cells and cause biological damage.
Properties of Beta Particles
- Beta particles are fast-moving electrons emitted from the nucleus of an atom and represented as
- Although β-particles and cathode rays are both fast-moving electrons, they differ in their origin. β-particles are given out from the nucleus, while the cathode rays are given out from the orbital electrons.
- The speed of beta particles is of the order of 108 m s−1(but less than 3 × 108 m s−1).
- Beta particles ionise the gas through which they pass. Their ionising power is roughly 1/100 times that of α-particles but nearly 100 times that of γ-radiation.
- Their penetrating power is more than that of α-particles.
- Beta particles are negatively charged, so they get deflected by electric and magnetic fields. The deflection of a β-particle is more than that of an α-particle because a β-particle is lighter than an α-particle.
- They affect a photographic plate.
- They cause fluorescence on striking a fluorescent material.
- They produce X-rays when they are stopped by metals of high atomic number and high melting point such as tungsten.
Properties of Gamma Particles
- They are electromagnetic waves such as X-rays and light, but they differ from X-rays and light in wavelength.
- The speed of γ-radiations is the same as the speed of light.
- The ionising power of γ-radiations is low. It is 10−4 times that of α-particles and 10−2times that of β particles.
- Their penetrating power is high. It is about 104 times that of α-particles and 102 times that of β-particles.
- Like X-rays and light, gamma radiations are not deflected by electric and magnetic fields because they are not charged particles.
- They affect a photographic plate.
- They cause fluorescence when they strike a fluorescent material.
- Like X-rays, γ-radiations are also diffracted by crystals.
- Gamma radiations are useful in the treatment of cancer.
Changes within the Nucleus in Emission of Alpha, Beta and Gamma Particles
Alpha Emission
If an unstable nucleus contains more neutrons than the number of protons, then it may emit two protons and two neutrons tightly bound together in a single particle, known as an alpha particle. A stream of α-particles is called α-rays.
Beta Emission
In emitting a β-particle, the number of nucleons in the nucleus remains the same, but the number of neutrons is decreased by one and the number of protons is increased by one.
In other words, by the emission of a β-particle, the mass number A does not change, but the atomic number Z is increased by one.
Gamma Emission
The γ-rays take no mass and no electric charge from the nucleus, i.e. no neutrons or protons are lost, and hence, the nucleus does not decay into a different nucleus, i.e. there is no change in the mass number A and atomic number Z of the nucleus in gamma emission.
Here, the star (*) indicates the excited state of the nucleus.
Uses of Radioactivity − Radio Isotopes
Medical Use
- Diseases such as leukaemia and cancer are cured by radiation therapy. Radiations from cobalt-60 (~Co) are used to treat cancer by killing the cells in the malignant tumour of the patient.
- The salts of weak radioactive isotopes such as radio-sodium chloride, radio-iron and radio-iodine are used for diagnosis. Such radio isotopes are called tracers.
- γ-rays emitted by radio isotopes are used to sterilise bandages, dressings, syringes and other equipment to make them free of germs. This method is quicker, more reliable and cheaper than sterilisation by heat.
Scientific Use
- Alpha particles emitted from radio isotopes are used as projectiles for nuclear reactions. The scattering of alpha particles from the nucleus helps in estimating the size of the nucleus and in understanding the nature of nuclear forces.
- The radioactive tracers are used in agricultural science to study the growth of plants with respect to the chemical manure used.
- The age of rocks and hence buried plants is estimated by the study of the rate of decay of 146Cin the remains of dead plants. The process is called carbon dating.
Industrial Use
- Radio isotopes are used as fuel for atomic energy reactors.
- Radio isotopes are used by engineers in factories to avoid the accumulation of charge on moving parts due to friction.
- The ionising effect of radiations from radio isotopes is used in making certain luminescent signs.
- The thickness of paper, plastic and metal sheets is controlled during manufacture when the penetrating power of β-radiations emitted from radio isotopes is known.
Harmful Effects and Safety Precautions of Radiation
Harmful Effects of Radiation
(a) Radioactive fallout from nuclear plants and other sources
(b) Disposal of nuclear waste
Biological Effects of Nuclear Radiations are of Three Types
- Short-term recoverable effects such as diarrhoea, sore throat, loss of hair and nausea
- Long-term irrecoverable effects such as leukaemia and cancer
- Genetic effects
Safety Rules for Handling Radioactive Materials
- Personnel should put on special lead-lined aprons and lead gloves.
- They should handle radioactive materials with long lead tongs.
- The safety limit for each type of radiation is known; therefore, care must be taken so that no one is exposed beyond the safety limit in any case.
- Radioactive substances must be kept in thick lead containers with a narrow opening, so as to stop radiations coming out from other directions.
Safety Measures in Establishment of Nuclear Power Plants
- The nuclear reactor of the power plant must be shielded with lead and steel walls so as to stop radiations from escaping out to the environment during its normal operation.
- The nuclear reactor must be housed in an airtight building of strong concrete structure which can withstand earthquakes, fires and explosion.
- There must be a back-up of the cooling system for the reactor core, so that in the case of failure of one system, the other cooling system can take its place, and the core is saved from over-heating and melting.
Safe Disposal of Nuclear Waste
- The radioactive material after its use is known as nuclear waste. The nuclear waste obtained from laboratories, hospitals, scientific establishments or power plants must be kept in thick casks and then they must be buried in specially constructed deep underground stores. These stores must be made far from populated areas. The casks can also be buried in useless mines, and these mines must be sealed after storing the casks.
Nuclear Energy
- The total sum of masses of product nuclei is always less than the total sum of the masses of reactant nuclei in a nuclear change due to radioactive phenomena. This implies that there is a loss in mass.
- In 1905, Einstein suggested that mass and energy are interchangeable. The energy E released due to the loss in the mass Δm is E = Δmc2, where c is the speed of light.
- 1 kg mass is equivalent to 9 × 1016 J or 2.5 × 1010 kWh of energy. 1 a.m.u. of mass is equivalent to 931 MeV of energy.
Nuclear Fission
- Nuclear fission is the process in which a heavy nucleus is splits into two light nuclei nearly of the same size by bombarding it with slow neutrons. In each fission reaction, a tremendous amount of energy of approximately 190 MeV is released.
- It was first observed by German Scientist Otto Hahn and Fritz Strassmann in 1983 in nuclear fission heavy nucleus splits into two smaller nuclei with liberation of energy.
- When uranium with Z = 92 is bombarded with neutron, it splits into two fragments namely barium (Z = 56) and krypton (Z = 36) and a large amount of energy is released which appears due to decrease in the mass.
Nuclear Energy Obtained in One Fission Reaction of 23692Unucleus
Let us consider the fission reaction of
We consider the mass of neutron = 1.01 a.m.u., mass of uranium−235 nucleus = 234.99 a.m.u., mass of barium−144 nucleus = 143·87 a.m.u., mass of krypton−89 nucleus = 88·90 a.m.u.
Loss in mass in fission reaction of one nucleus is
But from the mass-energy equivalence E = (Δm)c2
∴ Energy released E = 0·20 × 931 MeV = 190 MeV
Thus in the fission of one 23592U nucleus, nearly 190 MeV energy is released.
The major part of this energy is obtained in form of the kinetic energy of the fragments obtained from the fission and the remaining part is obtained in the form of the kinetic energy of the neutrons emitted, γ-rays, heat and light.
Controlled and uncontrolled chain reactions
- The energy obtained from the nuclear fission continuously increases. This chain reaction leads to a strong explosion of entire uranium in a very short interval of time along and also tremendously high amount energy is released which can be very harmful. This is the uncontrolled chain reaction.
- The basic principle of a nuclear bomb is based on uncontrolled chain reaction.
- If the chain reaction is controlled by absorbing some of the neutrons emitted in the fission process by means of moderators like graphite, heavy water, etc. then the energy obtained in fission can be utilized for the constructive purposes. A nuclear reactor works on this principle.
Uses of energy released in the process of fission
- Constructive Use: In nuclear reactor where the rate of release of energy is slow and controlled which is used to generate the electric power
- Destructive Use: In nuclear bomb where the energy released is fast and uncontrolled
Nuclear Fusion
- Nuclear fusion is the process in which two light nuclei combine to form a heavy nucleus and release a huge amount of energy. This is because the mass of the product nucleus is less than the sum of masses of the two combining nuclei.
- According to the mass−energy equivalence relation this loss in mass is released in form of energy E = (Δm)c2
Example: When two deuterium nuclei (21H) fuse, nucleus of helium isotope 32He is formed and 3.3 MeV energy is released. This helium isotope again fused with one deuterium nucleus to form a helium nucleus 42He and 18.3 MeV of energy is released in this process.
- Thus in all, three deuterium nuclei fuse to form a helium nucleus with a release of 21·6 MeV energy. A part of this energy is obtained in form of the kinetic energy of neutron and proton.
- When two nuclei approach each other, due to their positive charge, the electrostatic force of repulsion between them becomes too strong that they do not fuse. Thus, nuclear fusion is not possible at ordinary temperature and ordinary pressure.
Distinction between the Nuclear Fission and Nuclear Fusion
|
Nuclear Fission |
Nuclear Fusion |
|
A heavy nucleus splits in two nearly equal light fragments when bombarded with neutrons. |
Two light nuclei combine to form a heavy nucleus at very high temperature and high pressure. |
|
Possible at very high temperature and high pressure. |
Possible only at a very high temperature (≈107 K) and a very high pressure. |
|
Nearly 190 MeV of energy is released in one fission reaction. |
Nearly 24·7 MeV of energy is released in one fusion reaction. |
|
For same mass, the amount of energy released in fission is much lower than energy released in fusion. |
For same mass, the release of energy in fusion is way higher than that of fission reaction. |
|
The fissionable substance being radioactive gives out harmful radiations and thus creates problem in disposal of its waste. |
The fusionable substance is not radioactive, so it does not give out any harmful radiation and disposal of its waste is also not difficult. |
|
The fissionable substance is found within limit. |
The fusionable substance is found in abundance. |
|
Fission process can be controlled. Nuclear reactor is based on the controlled fission reaction. |
Fusion reaction cannot be controlled. This is why fusion reactor could not be constructed so far. |
|
Nuclear bomb is based on the uncontrolled fission reaction. |
Hydrogen bomb is based on the uncontrolled fusion reaction. |