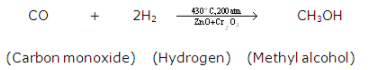Frank Solutions for Chapter 5 Physical and Chemical Changes Class 9 Chemistry ICSE
1. What is a physical change?
Answer
A physical change is a temporary change in which no new substance is formed and the composition or identity of the substance is not altered although certain specific physical properties may be changed.
2. What is a chemical change?
Answer
A chemical change is a permanent change in which the original substance gives rise to one or more substances with different properties.
3. What are the exothermic and endothermic reactions ?
Answer
The reactions in which heat is evolved are called exothermic reactions while the reactions in which heat is absorbed are called endothermic reactions.
4. Indicate which of the following statements are true and which are false.
(a) A change, whether physical or chemical is always permanent.
(b) Photosynthesis in plants is a physical change.
(c) The mass of a substance does not change on burning.
(d) Carbohydrate burn in air to give carbon and water.
(e) A chemical change is always accompanied by the evolution or absorption of heat.
Answer
(a) False
(b) False
(c) False
(d) False
(e) True
5. Why do we call the burning of paper as chemical change ?
Answer
When wood or paper is burnt in air, it gives carbon dioxide and water leaving behind a little ash.
[C6(H2O)5]n + 6nO2 → 6nCO2 + 5nH2O + Heat
Wood Oxygen Carbon dioxide Water
We call it as chemical change because –
- In above reaction new substances are formed. Impurities which are non-volatile, remain behind.
- The change is permanent.
- Heat and light energies are given out.
- Mass of wood get changed but the total mass remains the same.
6. What are the possible conditions for a chemical change ?
Answer
Possible conditions for a chemical change are –
- One or more new substance is formed during reaction.
- The change occurring during the reaction is permanent.
- The mass of the substance undergoing a chemical change is generally altered.
- Chemical change involves making and breaking of bond.
7. What is double decomposition? Give two example.
Answer
Reactions in which both the reactants exchange their radicals to give new compounds are called double displacement or double decomposition reaction.
The general reaction can be written as : -
A+B- + C+D-→ A+D- + C+B-
The two examples of double decomposition reaction are : -
(a) Precipitation reactions- Reactions involving the formation of a precipitate. Sodium chloride and silver nitrate react to form an insoluble white precipitate of silver chloride.
For example:
NaCl + AgNO3 → AgCl (White ppt.) + NaNO3
(b) Neutralisation reaction- Reactions in which acids and bases mix together, forming compounds i.e., salt in water.
For example:
9. State what energy change accompanies the following chemical reaction ?
(a) Air is passed over white hot cake.
(b) Steam is passed over white hot coke.
Answer
(a) Exothermic reaction
(b) Endothermic reaction
10. What the major distinctions between physical and chemical changes ?
Answer
11. What is photochemical reaction?
Answer
The chemical reactions which occur with the absorption of light energy are called photochemical reactions.
Examples:
Decomposition of silver nitrate takes place in the presence of light.
12. How can you prove experimentally that magnesium gains weight on burning in air?
Answer
As the burning substance combines with oxygen, the total mass of the products should be greater than that of the burning substance. For example, when, magnesium is burnt, a new substance magnesium oxide is formed, whose weight is greater than that of original magnesium.
Experiment: A crucible is weighed containing about 0.5 gm of magnesium. Now crucible is heated. When magnesium begins to burn, the lid is put back on the crucible and the lid is occasionally raised to allow air to enter and burn the magnesium such that no product is lost. When, all magnesium has been burnt up, the crucible is allowed to cool and then on weighing it we observe that there is gain in weight.
13. Give three differences between respiration and burning ?
Answer
14. Name three conditions necessary for burning ?
Answer
Three conditions necessary for burning are-
- The substances to be burnt must be combustible.
- A supporter of combustion such as air or oxygen must be present.
- A combustible substance must be heated to its ignition temperature.
15. Give balanced equations where possible or where it is not possible, explain by means of examples.
(a) A reaction which gives cut heat.
(b) A reversible reaction.
(c) A reaction with a solid and a gas which produces heat and light.
(d) A reaction which takes place with the help of sunlight.
(e) A reaction which is brought about by an electric current.
Answer
(a) A reaction which gives out heat is called an exothermic reaction. For example:-
Burning of coal in air is an example of exothermic reaction.
C + O2 → CO2 + heat
(b) A reaction which proceeds in both forward as well as backward directions. They are indicated by the sign ’→’.
3Fe + 4H2O → Fe3O4 + 4H2
(c) 2Mg + O2 → 2MgO + light + heat
(d) A reaction which takes place with the help of sunlight.
(e)
16. Give balanced equation for the action of heat on the following :
Silver nitrate, Copper (II) nitrate, Aluminium hydroxide, Silver carbonate and Potassium nitrate
Answer
17. Oxidation and reduction go hand in hand. Explain.
Answer
When oxidation occurs there is a loss of electrons but simultaneously there is a gain of electrons by other species which is called reduction. These both process occur simultaneously so we can say that both oxidation and reduction go hand in hand and such reactions are known as redox reaction.
18. Name the substances which are oxidized and which are reduced in the following reactions.
(a) Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
(b) Ag2O + H2O2 → 2Ag + H2O + O2
Answer
(a) Copper is oxidized to copper sulphate while sulphur in sulphuric acid is reduced to sulphur dioxide.
(b) Silver in silver oxide is reduced to silver while oxygen in hydrogen peroxide is oxidised to molecular oxygen.
19. Name and define the four important types of chemical reactions.
Answer
Four types of chemical reactions are –
- Direct combination or Synthesis reactions
- Decomposition reactions
- Displacement reactions
- Double decomposition reactions
(i) Direct combination or Synthesis reactions– When two or more elements combine to form a compound, the process is called synthesis. It may be brought about by the action of heat, electricity or pressure,
For example:
(a) Where two elements combine to form a new compound.
(b) Where an element and a compound combine to form a new compound.
(a) Decomposition by heat:
(b) Decomposition by electricity:
(iii) Displacement reactions– A reaction in which one element displaces another element from its compound to form a new compound is called a displacement reaction.
For example:
A general reaction : -
A+ B- + C+D- → A+ D- + C+B-
For example :
(a) Precipitation reaction
Nacl + AgNO3 → AgCl ↓ + NaNO3
(b) Neutralisation reaction
NaOH + HCl → NaCl + H2O
20. Give a possible explanation for the following observations :
(a) Gold is found free (uncombined) in nature.
(b) Compounds of sodium do not decompose on heating.
(c) Silver articles turn black on prolonged exposure to air.
(d) When ammonium nitrate is dissolved in water contained in a beaker, the beaker becomes cold.
(e) When magnesium is dissolved in hydrochloric acid contained in a beaker, the beaker becomes hot.
Answer
(a) In activity series, Gold is placed almost at the end of the series since it is least reactive. So, it does not tend to react with other elements easily and thus found free in nature.
(b) For most of the ionic compounds the lattice enthalpy is very high therefore, they do not easily decompose on heating.
(c) On prolonged exposure to air silver react with oxygen present in air to form silver oxide which is black in colour.
Ag + O2 → Ag2O
Silver (grey) Silver oxide (black)
(d) The reaction between ammonium nitrate and water is endothermic. It takes away heat from the beaker. So, beaker becomes cold.
(e) The reaction between magnesium and hydrochloric acid is an exothermic reaction which gives heat to the beaker and thus beaker becomes hot.
Mg + 2HCl → MgCl2 + H2 + Heat
21. As a candle burns, first the physical change then chemical change takes place. Explain.
Answer
A candle is a tick of paraffin wax with cotton wick. As a candle burns wax melts and trickles down. It gets solidified shortly. This is a physical change. Parafffin wax is a mixture of hydrocarboons. When wick catches fire, paraffin wax melts, evaporates and burns in air like any hydrocarbons to give carbon dioxide and water. This is a chemical change.
22. Fill in the blanks:
(a) Dissolving common salt in water is an example of …….. change.
(b) Dissolving zinc in dil. H2SO4 is an example of .……. Change.
(c) A ………. Change is one in which one or more different substances are formed.
(d) A ……… change can easily be reversed.
Answer
(a) physical
(b) Chemical
(c) chemical
(d) Physical
23. Give two examples for each of an oxidation and reduction type of reactions, which take place on a large scale.
Answer:
Two examples are :
- Burning of wood-carbon get oxidized and oxygen gets reduced.
- Rusting – In it iron is oxidized.
24. Give an example for each of the following:
(a) An oxidizing agent not containing oxygen.
(b) A substance which acts as both oxidizing and reducing agent.
(c) A carbonate stable towards heat.
(d) A compound decomposed by light.
(e) A positive catalyst.
Answer
(a) Chromium (VI)
(b) Hydrogen peroxide
(c) Barium carbonate.
(d) Silver nitrate
(e) Manganese dioxide
25. What is meant by the term ignition temperature? How is it related to burning ?
Answer
Ignition temperature – Ignition temperature is the lowest temperature up to which temperature of a substance must be raised so that it catches fire. A combustible substance must be heated to its ignition temperature for burning.
26. Give three differences between respiration and combustion of fuels ?
Answer
27. Show that a physical change can easily be reversed.
Answer
On heating few crystals of iodine in a test tube, the grey crystals sublimes and dense violet fumes are seen. On cooling, the vapours again form the crystals. So, a physical change can be reversed.
28. When hydrogen burns in air, the change is chemical. Give two reasons to support this assertion.
Answer
When hydrogen burns in air, formation of water occurs.
2H2 + O2 → 2H2O
Two reasons are:
- Water is formed as a product which is different from hydrogen and oxygen. Mass of water is different from either hydrogen or oxygen but the total mass of the substance involved in the chemical reaction remains same.
- The change is permanent and cannot be reversed by reversing the conditions that initially caused the change to occur.
29. Freezing of water to ice and evaporation of water are both physical change. Explain.
Answer
When water is freezed and evaporated, these both are physical changes because –
- The change is temporary and reversible.
- No new substance is formed and the chemical composition of the original substance remains the same.
- Mass of the substance remains unchanged.
- The amount of energy required to bring about a physical change is generally equal to the amount of energy required to reverse the change. Hence, there is not energy change involved.
30. What is synthesis? What kind of chemical reaction is synthesis ? Support your answer by an example ?
Answer
When two or more elements combine to form a compound, the process is called synthesis. It may be brought about by the action of heat, electricity or pressure. Reactions in which direct combination of chemical substances occur are called as synthesis.
Example:
- (a) When two elements combine to form a new compound.
H2 + Cl2 → 2HCl - Where an element and a compound combine to form a new compound.
2CO + O2 → 2CO2 - Where two compounds combine to form new compounds.
NH3 + HCl → NH4Cl
31. Air is necessary for burning. Comment.
Answer
Air is necessary for burning. Incorrect amount of air in fuel combustion accounts for the largest losses in combustion system. If the fuel does not get enough air for combustion it will generate smoke and a potentially unhealthy mixture of gas products.
32. (a) What are combustible and non-combustible substances ?
(b) Name two substances other than oxygen that support combustion.
Answer
(a) Combustible substances - The substances that catch fire and burn easily.
Example: Wood, Charcoal, petrol, Kerosene etc.
Non–combustible substances – Substances which cannot burn in air or oxygen are called as non-combustible substances.
Example: Nitrogen gas, carbon dioxide etc.
(b) Two substances other than oxygen that support combustion are -
- Hydrogen
- Nitrogen
33. State two important processes, which :
(a) release CO2 into the atmosphere
(b) remove CO2 from the atmosphere
Answer
(a) (i) Burning of coal in air releases CO2 in air.
(ii) Respiration releases carbon dioxide and water vapours.
(b) (i) Photosynthesis removes CO2 from the atmosphere. Plants take carbon dioxide from the atmosphere in the presence of sunlight and use it to synthesise glucose with the liberation of oxygen.
(ii) Some man made chemical activities such as setting of mortar also use atmospheric carbon dioxide and helps in removing carbon dioxide.
34. What would be the effect on burning if the proportions of nitrogen and oxygen in the air were reversed ?
Answer
Nitrogen is inert in nature and does not support combustion while oxygen supports combustion. If proportions of nitrogen and oxygen in the air were reversed then the rate of combustion of substances will increase.
35. Give an experiment to show a physical change?
Answer
Heating of sulphur –
If some powdered sulphur is heated gently in a glass test tube, it melts to a pale yellow liquid. Flame is removed to stop heating, it is quickly changed back to solid sulphur.
36. What is activity series ? What is the role it plays in displacement reactions ?
Answer
Activity series – The arrangement of the metals in the decreasing order of their chemical reactivity is called the activity series.
In displacement reactions, a more reactive element (metal or non-metal) displaces a lesser reactive element from its compound. With the help of the activity series, it is possible to predict which metals will displace other metals from their solutions.
37. How the balance of oxygen and carbon dioxide is maintained in nature ?
Answer
Balance of oxygen and carbon dioxide is maintained in nature because there is a natural oxygen cycle and a natural carbon cycle operating all the time by which the desired proportions of the two gases in the air are maintained. This is also known as carbon dioxide – oxygen cycle.
38. Balance the following equations and also state the type of reaction that is taking place :
(b) ZnCO3 → ZnO + CO2(c) Zn + FeSO4 → ZnSO4 + Fe
(d) Mg + HCl → MgCl2 + H2
(e) P2O5 + H2O → H3PO4
(f) KI + Cl2 → KCl + I2
(g) Cu(NO3)2 → CuO + NO2 + O2
(h) ZnSO4 + NaOH → Zn(OH)2 + Na2SO4
(i) Na + H2O → NaOH + H2
(j) NaOH + H2SO4 → Na2SO4 + H2O
Base Acid
(k) Fe + S → FeS
(l) Mg(HCO3) → MgCO3 + H2O + CO2
(m) CaO + H2O → Ca(OH)2
(n) CaCl2 + NH4OH → Cu(OH)2 + NH4Cl
(o) Mg + N2 → Mg3N2
Answer
(a)
(b) ZnCO3 → ZnO + CO2
Decomposition reaction
(c) Zn + FeSO4 → ZnSO4 + Fe
Displacement reaction
(d) Mg + 2HCl → MgCl2 + H2
Displacement reaction
(d) Mg + 2HCl → MgCl2 + H2
Displacement reaction
(e) P2O5 + 3H2O → 2H3PO4
Combination reaction
(f) 2KI + Cl2 → 2KCl + I2
Displacement reaction
(g) 2Cu(NO3)2 → 2CuO + 4NO2 + O2
Decomposition reaction
(h) ZnSO4 + 2NaOH → Zn(OH)2 + Na2SO4
Double decomposition reaction
(i) 2Na + 2H2O → 2NaOH + H2
Displacement reaction
(i) 2NaOH + H2SO4 → Na2SO4 + 2H2O
Displacement reaction
(j) 2NaOH + H2SO4 → Na2SO4 + 2H2O
Base Acid
Double decomposition reaction
(k) Fe + S → FeS
Combination reaction
(l) Mg(HCO3)2 → MgCO3 + H2O + CO2
Decomposition reaction
(m) CaO + H2O → Ca(OH)2
Combination reaction
(n) CaCl2 + 2NH4OH → Ca(OH)2 + 2NH4Cl
Double decomposition reaction
(o) 3Mg + N2 → Mg3N2
Combination reaction
39. Write short note on carbon dioxide cycle.
Answer
Carbon dioxide from the atmosphere enters the plant through photosynthesis, where carbohydrates are produced. From green plants, the carbon in the form of carbohydrates, etc. enter the animal and human bodies. The atmospheric carbon dioxide gets dissolved in oceans by diffusion. Marine algae and photosynthetic bacteria obtain carbon dioxide from water.
Carbon dioxide returns to the atmosphere by respiration, combustion of fossil fuels like coal, wood, petroleum etc., weathering of rocks, volcanic eruptions etc.
40. Write about oxygen cycle.
Answer
The oxygen cycle is the biogeochemical cycle that describes the movement of oxygen within atmosphere, biosphere and lithosphere. The main source of atmospheric oxygen is photosynthesis, which produces sugars and oxygen from carbon dioxide and water.
Oxygen also comes from photolysis.
Then oxygen is taken away from atmosphere by plants and animals. It is also consumed from atmosphere by chemical weathering i.e., oxidation of exposed minerals and rocks.
4FeO + O2 → 2Fe2O3